designed with purpose
Sacroiliac joint disruptions and degenerative sacroiliitis are chronic conditions that can cause pain in the lower back, hips and legs. SacroFuse helps eliminate this pain by fusing or fixating the sacroiliac joint, reducing irregular movement by stabilizing the joint to alleviate much of the pain. This is a less exposure outpatient procedure that can dramatically improve a patient’s quality of life.
Featured Videos
Features & Benefits
Polyaxial Washer Contours to the Facet
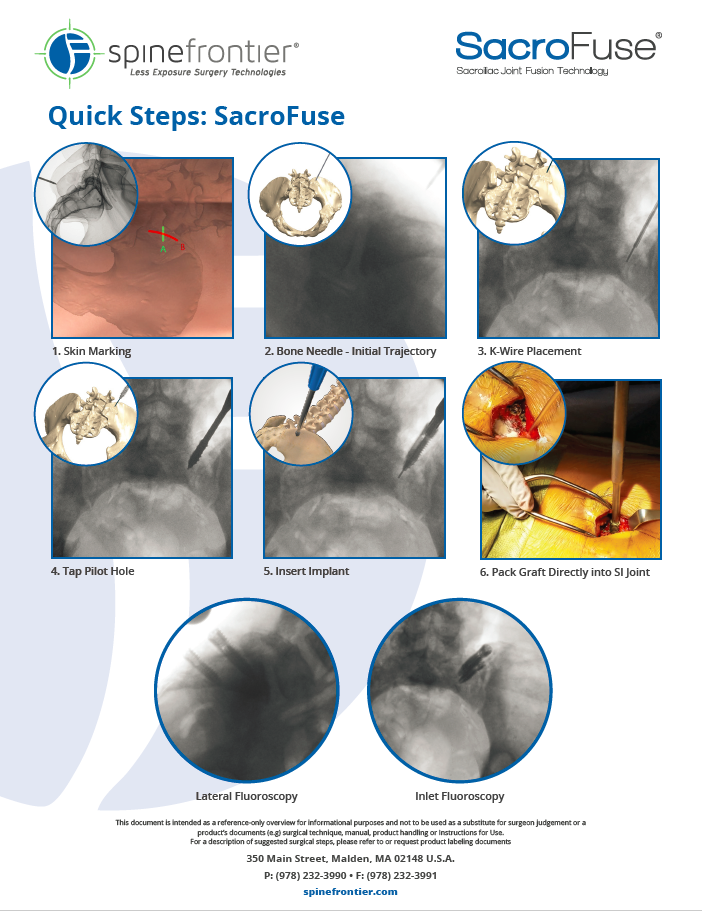
SacroFuse screws are inserted from a posterior approach, via straightforward, cannulated instrumentation. Cortical and cancellous thread profiles ease insertion and optimize purchase for differing bone densities.
Opposing Teeth Minimize Backout
The inner fusion network funnels bone product from the insertion feature through the implant, directly into the fusion channels. Fusion channels self-harvest autograft during insertion, creating three equally spaced fusion columns and aiding fixation through the ilium, SI joint and sacrum.
Lag Screws Provide Compression
Bulleted tip and self-cutting features optimize and enable pilot hole creation immediately following the bone needle, eliminating the need for supplementary instrumentation. If desired, this can be a simple, two step process with bone needle/trajectory establishment and screw insertion.
Resources
Indications
The SpineFrontier Lumbar Interbody Fusion Device System (Dorado IBC, Dorado PLIFT, Dorado ELIFT, Dorado ALIFT, Dorado TILT, Dorado TLIFT, Dorado Wide, and Ursa S-LIFT) is intended for intervertebral body fusion of the spine of skeletally mature patients, using autogenous bone graft to facilitate fusion. The device is indicated for use in patients with degenerative disc disease (DDD) at one or two contiguous spinal levels from L2-S1. These DDD patients may also have up to Grade I spondylolisthesis or retrolithesis at the involved level(s).
Degenerative disc disease is defined as discogenic back pain with degeneration of the disc confirmed by history or radiographic studies. These patients should be skeletally mature and have had six months of non-operative treatment.The SpineFrontier Lumbar Intervertebral Body Fusion Device System is intended to be used with supplemental spinal fixation system(s) cleared for use in the lumbar spine.