Designed With Purpose
P-LIFT is a revolutionary posterior lumbar interbody designed and engineered by SpineFrontier for use with Less Exposure Surgery techniques. It features a sleek low-profile design, which aids in sparing patient anatomy during surgery. Its large graft window, robust inserter platform, and cannulated design offer security and provide each surgeon with the confidence that we at SpineFrontier pride ourselves on.
Features & Benefits
Large Graft Window
P-LIFT’s substantial graft cavity is designed with fusion in mind. Used in conjunction with bone graft, this centralized bone graft window supports bony fusion and patient stability.
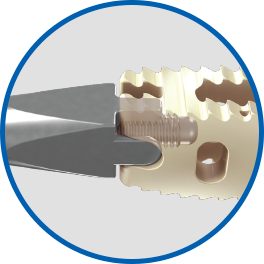
Secure Robust Inserter
Our interbody inserter platform is designed with simplicity and security in mind. This inserter provides surgeons with the convenience and confidence SpineFrontier is known for.
Lateral Interbody Windows
In addition to a large centralized graft cavity, our P-LIFT Interbody also contains graft windows on the walls of the interbody, designed to promote fusion. These graft windows are specially designed to promote fusion when used in conjunction with bone graft.
INDICATIONS
The SpineFrontier Lumbar Interbody Fusion Device System (Dorado IBC, Dorado PLIFT, Dorado ELIFT, Dorado ALIFT, Dorado TILT, Dorado TLIFT, Dorado Wide, and Ursa S-LIFT) is intended for intervertebral body fusion of the spine of skeletally mature patients, using autogenous bone graft to facilitate fusion. The device is indicated for use in patients with degenerative disc disease (DDD) at one or two contiguous spinal levels from L2-S1. These DDD patients may also have up to Grade I spondylolisthesis or retrolithesis at the involved level(s).
Degenerative disc disease is defined as discogenic back pain with degeneration of the disc confirmed by history or radiographic studies. These patients should be skeletally mature and have had six months of non-operative treatment.The SpineFrontier Lumbar Intervertebral Body Fusion Device System is intended to be used with supplemental spinal fixation system(s) cleared for use in the lumbar spine.